Efficient Laser Process Yields Crystalline Iridium Oxide Nanoparticles with promising Catalytic Properties
7. July 2025 - In a step toward advancing sustainable energy technologies, researchers have developed a laser-induced nanoscale engineering process that enables the controlled synthesis of ultra-small, crystalline iridium oxide nanoparticles. These materials demonstrate outstanding activity and stability in the anode reaction of proton exchange membrane water electrolysis—a key process in green hydrogen production. By enabling more efficient use of scarce and costly catalyst materials, this approach supports strategic goals in resource conservation, energy transition, and the development of a resilient hydrogen economy.
The catalyst bottleneck: a trade-off between stability and activity
The electrochemical production of hydrogen is considered a key component of a climate-friendly energy future. Polymer electrolyte membrane water electrolysis (PEMWE) has great potential because it enables high current densities, fast dynamic reactions, and efficient hydrogen production at high pressures. However, the slow oxygen evolution reaction at the anode hinders efficiency, especially under PEMWE's extremely acidic conditions. Using suitable, stable catalysts is therefore a key challenge.
One of the few materials that remains stable under the harsh conditions of PEMWE is crystalline iridium oxide. However, due to its low natural availability and limited activity, more efficient atom utilization is required. Conventional high-temperature post-processing methods form the stable rutile phase but also promote the growth of larger particles, which reduces the catalytically active surface area and performance.
Innovative approach: Laser-induced nano oven process
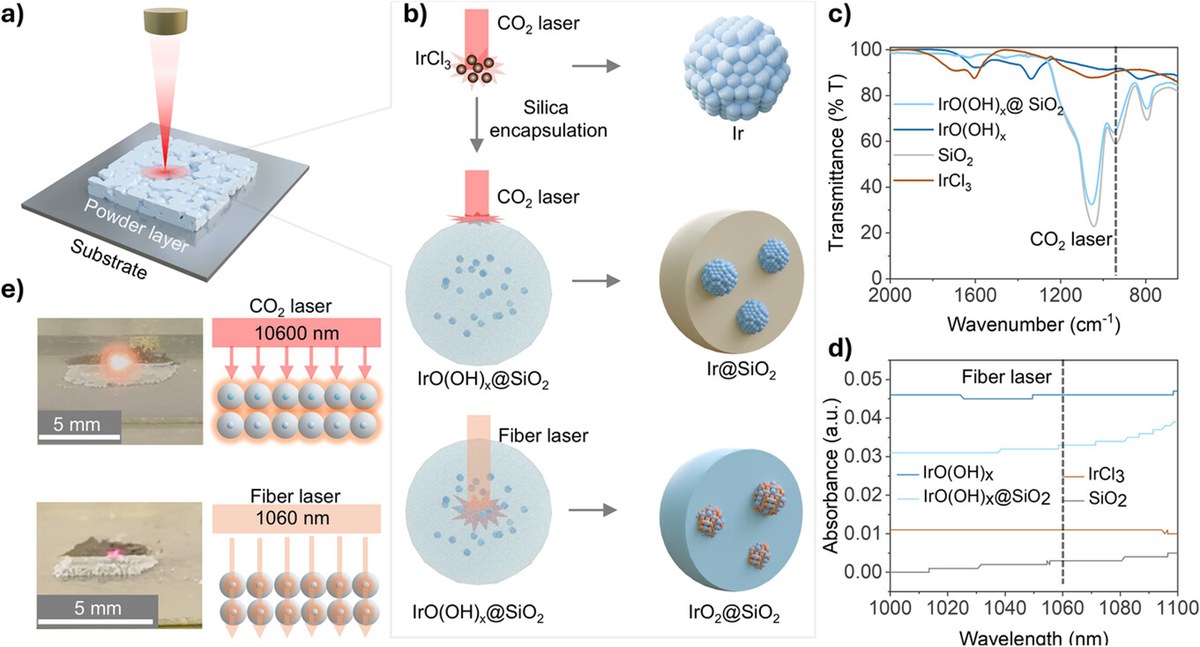
A research team from HI ERN, FAU, and TUM has developed a novel laser-based process for synthesizing ultra-small, crystalline iridium oxide nanoparticles. The method employs a silicon dioxide matrix as a nanoscale reaction chamber, effectively preventing particle agglomeration and enabling precise control over particle size. This results in IrO₂ nanoparticles just two nanometers in diameter, stabilized in the rutile crystal structure. These nanoparticles achieve a mass activity of 350 ± 15 A gIr⁻¹ at an overpotential of 300 mV, exceeding the activity of conventional ruthenium oxide and rivaling the performance of the best RuO₂-based catalysts. Real-time ICP-MS analysis in a channel flow cell shows that the laser-produced IrO₂ is significantly more stable than commercially available iridium oxide.
The results of this collaborative effort were recently published in the prestigious journal Angewandte Chemie and have far-reaching relevance for both research and application. They demonstrate a promising strategy for improving catalysts for the hydrogen economy. It also provides an approach for producing ultra-small, crystalline metal oxides under mild conditions, with potential applications far beyond electrochemical hydrogen production. Thus, the results contribute to technological progress in the energy transition and to a better understanding of nanoscale material processes.
Original publication
Laser-Induced Nanoscale Engineering of Iridium-Based Nanoparticles for High-Performance Oxygen Evolution
H. Wang, P. Pfeifer, W. Lai, A. Göpfert, S. Lim, W. Zhao, A. L. Morales, M. Goßler, M. Malinovic, P. Bhuyan, W. A. Parada, P. Nikolaienko, K. J. J. Mayrhofer, G. V. Fortunato, A. Hutzler, M. Ledendecker, Angew. Chem. Int. Ed. 2025, e202508589.
https://doi.org/10.1002/anie.202508589