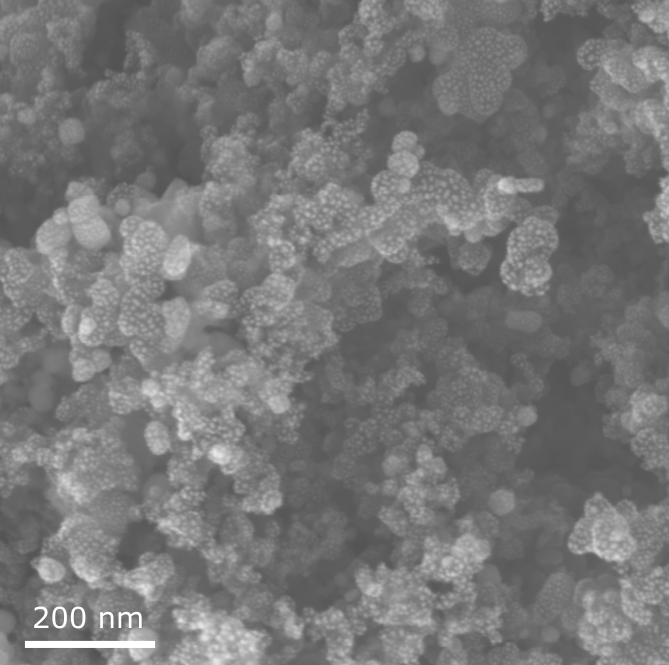
Catalysts for fuel cells und electrochemical synthesis reactors
Involved researchers: Moritz Valeske, Ramesh Pokhrel

Hydrogen implementation is facing societal resistance due to safety concern and high management costs of molecular hydrogen. To increase its energy-density, hydrogen is often stored and used in compressed (300 – 700 bar) or liquefied (- 253°C) states. One solution for overcoming this deficiency is the usage of liquid organic hydrogen carriers (LOHC) to store hydrogen in their chemical bonds. H2 can be discharged and charged from and to LOHCs by de/hydrogenation reactions. LOHCs are in a liquid state in wide temperature range at ambient pressure, and often inflammable. Thus using LOHCs facilitates not only the fuel management within the fuel cell systems, but also the storage and transportation of the fuel. Therefore, cost reduction and safer operation are expected for these systems. However, de/hydrogenation reactions are happened at high temperature (250 – 350 °C) and thus energy-extensive and inflexibly operative. We develop alternative way for the reactions via de/hydrogenation in electrochemical reactors, which is conducted at temperature < 100 °C. The systems are anticipated to be energy-efficient and dynamically operational. Examples of LOHC systems are isopropanol/acetone and methyl cyclohexane/toluene couple. The isopropanol/acetone couple can be deployed directly in fuel cell as direct isopropanol fuel cell (DIFC), or indirectly via de/hydrogenation in an electrochemical reactor. We design and develop the catalysts for these reactors