Non-precious hydrogen evolution reaction (HER) catalysts
Involved researchers: Stephan Ruck
HER catalyst for PEMWE
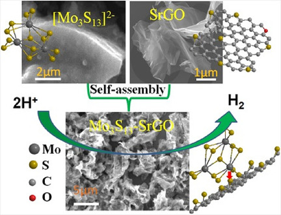
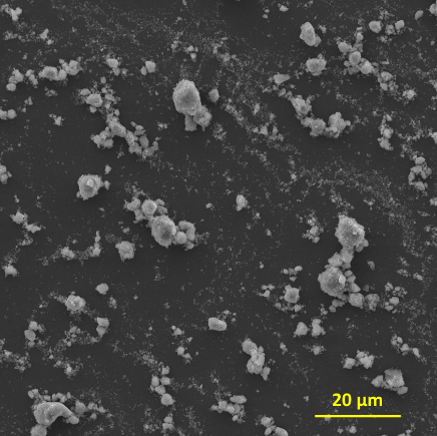
Pt nanoparticles supported on carbon black is currently the best catalyst for the hydrogen evolution reaction (HER) in the cathode. The scarcity and high cost motivates the seek for non-precious alternative catalysts. A biomimetic study inspired the idea of using molybdenum sulfides (MoSx) as cheap and abundant HER catalysts in corrosive acidic conditions. Further development has proven it to be the most promising candidate to replace Pt in acidic HER. The catalytic active sites of MoSx are proven at its edge structures. Therefore, [Mo3S13]2- nanoclusters was synthesized to have the highest exposing edge sites for HER catalysis. To advance the practical application of this catalyst, we synthesized [Mo3S13]2- nanoclusters decorated graphene and carbon nanotube to form composite catalysts (Mo3S13-NCNT). These composite catalysts with better dispersibily and electrical conductivity are more suitable for device applications.
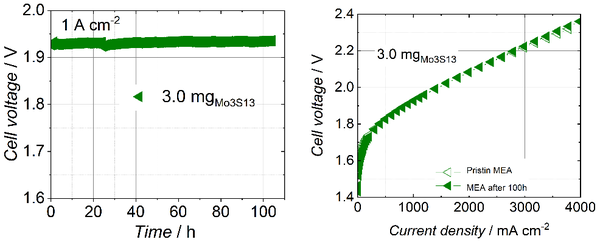
For full-cell PEMWE test, the Mo3S13-NCNT hybrid catalyst was spray-coated on a porous carbon support to form freestanding electrodes. The cell exhibited a current density of 4 A cm-2 at a cell voltage of 2.36 V. This current density is the highest reported at the time of publication for a PEMWE using a non-noble metal based HER catalyst. In addition, a cell durability test by applying a constant current of 1 A cm-2 for 100 h showed a low degradation of 83 µV h-1 and an almost unchanged polarization curve after the current hold.
HER catalyst for anion exchange membrane water electrolyzers (AEMWE)
AEM water electrolysis has recently emerged as a cost-effective and efficient technology, which can be competitive to incumbent PEMWE and alkaline electrolysis. This is because AEMWE can deliver current density comparable with that of PEMWE on the one hand, and deploy non-noble catalysts and non-titanium porous transport layer and flow fields on the other hand. Moreover, recently stable AEM materials become available with potential cost reduction, making this technology more attractive. Nevertheless, a comprehensive design of high performing MEA with optimized interfaces using effective non-noble catalysts is still lacking. In this topic, we particularly focus on design and implementation of non-noble catalysts and catalyst layers for the HER at the cathode.
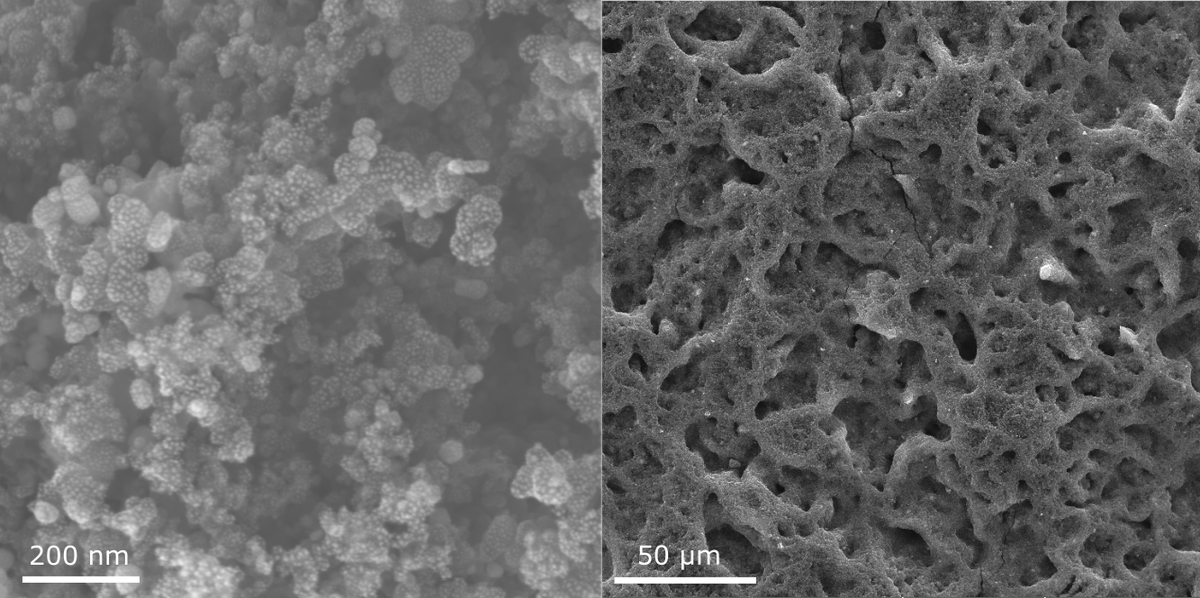
The HER at the cathode of AEMWE is relatively sluggish due to the basic conditions, compared to that of PEMWE. That is true even when Pt/C catalysts are used, and for non-noble catalysts its activity is largely compromised. Our catalyst solutions are therefore concentrated on doped non-precious catalysts for enhanced activity. Our research aim is to reduce or even omit the use of precious metal catalyst without compromising the performance of AEMWE
Further reading
Fabrication of a Robust PEM Water Electrolyzer Based on Non‐Noble Metal Cathode Catalyst: [Mo3S13]2− Clusters Anchored to N‐Doped Carbon Nanotubes, PKR Holzapfel, M Bühler, D Escalera‐López, M Bierling, FD Speck, K. JJ Mayrhofer, S. Cherevko, C. V. Pham, S. Thiele, Small 16 (37) (2020), 2003161.