The Surprising Flexibility of Ice at the Nanoscale
16 October 2025 - For the first time, researchers have been able to record the complex structures in ice that are formed during the freezing of liquid water. The results come from the first observations of the molecular structure of samples from thin samples of frozen water and were recently published in the journal Nature Communications.
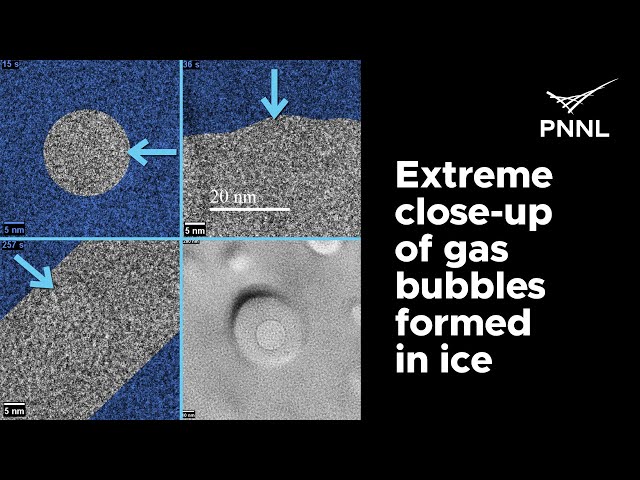
Even though ice forms in a perfectly hexagonal lattice, it is surprisingly flexible and malleable, which explains why ice so often has trapped gas bubbles. The discoveries were made by an international research team led by James De Yoreo from the Pacific Northwest National Laboratory (PNNL), which also included Andreas Hutzler and Birk Fritsch from the HI ERN. Their findings from the first-ever molecular-resolution observations of nanoscale samples of ice frozen from liquid water were recently published in the journal Nature Communications.
Until now, no one has been able to directly observe molecules of water undergoing the transition from liquid water to ice. That’s because the techniques scientists use to view individual atoms involve harsh conditions, including using high-energy radiation and removal of all air (vacuum sealing). To avoid these issues, the research team sandwiched liquid water between thin carbon membranes, which turned out to be the critical factor that led to this imaging breakthrough. With this approach, they developed a new technique denoted as cryogenic liquid-cell transmission electron microscopy, to follow the freezing process. In addition, the colleagues in Erlangen were able to provide information about the influence of electron irradiation on observed phenomena via radiolysis modeling.
The study results showed that when liquid water turns to solid ice, defects in its crystal structure or trapped gas bubbles don’t cause much strain to the ice crystal, which could cause fracturing. It adapts to the presence of defects surprisingly easy compared to other solids, like metals or minerals. The nature of water’s chemical bonds makes it extraordinarily flexible and malleable, even as solid ice. This new observation, combined with the crucial fact that ice is less dense than liquid water, are properties that support life on Earth, and especially in the sea.
The research could have profound implications for preserving deeply frozen (cryogenic) biological tissue samples, forecasting ice behavior for aviation and vehicle safety and understanding the movement of glaciers, among other areas of research.
Extreme close-up of gas bubbles formed on ice (Animation by Sara Levine | Pacific Northwest National Laboratory)
Original Publication
Du, J.S., Banik, S., Chan, H. et al.
Molecular-resolution imaging of ice crystallized from liquid water by cryogenic liquid-cell TEM. Nat Commun 16, 8342 (2025).
https://doi.org/10.1038/s41467-025-62451-0
Contact
Dr.-Ing. Andreas Hutzler
Team leader "Nanoanalysis of Electrochemical Processes"
Room 4009