Manufacturing of membranes and electrodes
We employ various techniques to produce membranes from ion exchange polymers and electrodes from catalyst nanoparticles, support, and binder. A key to efficient electrochemical energy conversion is the interface between the single layers, which is generated when they are sandwiched to form a membrane electrode assembly. Here, we provide an overview of the methods and devices that are used in our labs for manufacturing.
Spray coating
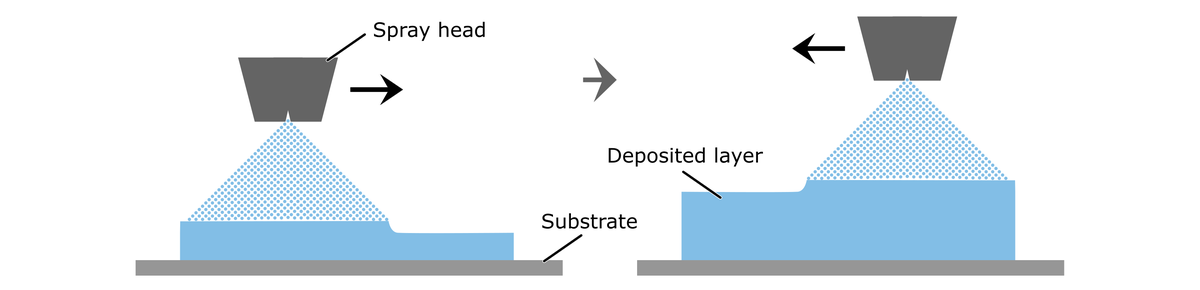

In spray coating, a low viscosity fluid (e.g., a polymer dispersion or catalyst ink) is finely atomized via an ultrasonic spray head. The fluid is deposited as a fine mist in consecutive spray runs onto a substrate, where it forms a thin, continuous layer after solvent evaporation. In this layer-by-layer approach, a spray coater produces a thin film of adjustable size and thickness. We employ ExactaCoat devices from SonoTek in our labs.
The main advantages of spray-coating are that the thickness of the films can simply be adjusted by the number of runs and the flow rate, and the solvent evaporation rate can be controlled by adjusting the substrate temperature. Furthermore, not only homogenous solutions can be sprayed, but also dispersions, which are fluids that contain a solid fraction, like catalyst and binder in electrode inks.
Doctor blade and Mayer rod coating
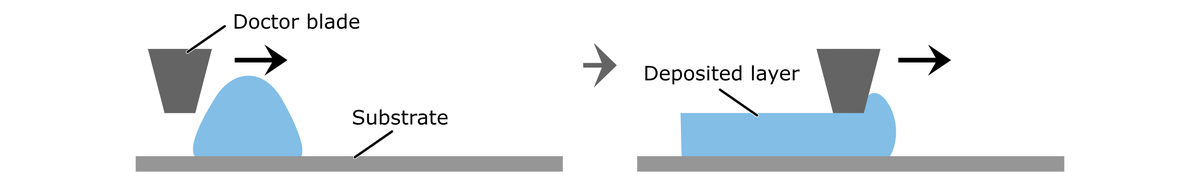
A doctor blade allows the fabrication of membranes and electrodes from polymer dispersions or catalyst inks. A line of the fluid is applied on a substrate, e.g., a Teflon sheet, a glass plate, or a gas diffusion layer. The doctor blade then moves at a user-defined gap height and speed along the substrate to coat it with the fluid.
One advantage of a doctor blade is that the layer production is typically faster than by spray coating because the thin films can be fabricated with a single run. A relevant difference to spray coating is that doctor blading processes fluids with a high viscosity, whereas inks with low viscosities are needed for spray coating.
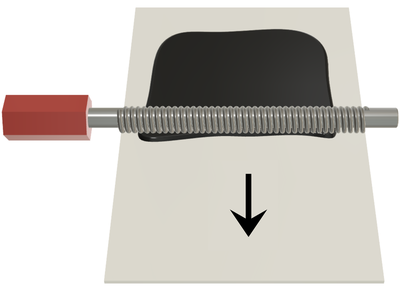
The Mayer rod technique is used to manufacture electrodes (layers of catalyst materials). The working principle is similar to the doctor blade. The main difference is the grooved structure of the Mayer rod. The depth and the distance of the grooves determine the thickness of the wet film. Furthermore, the presence of the grooves supports a uniform coating.
Electrospinning
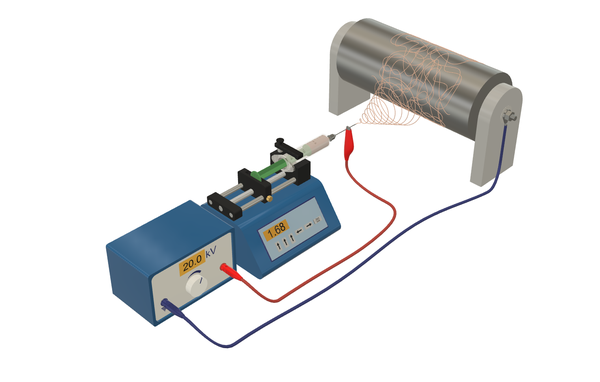
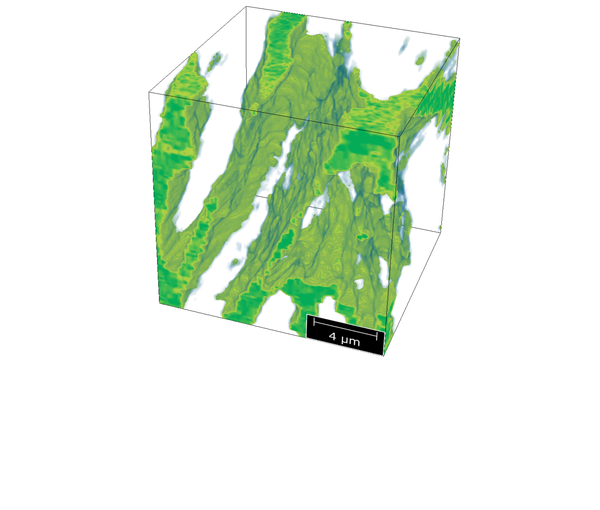
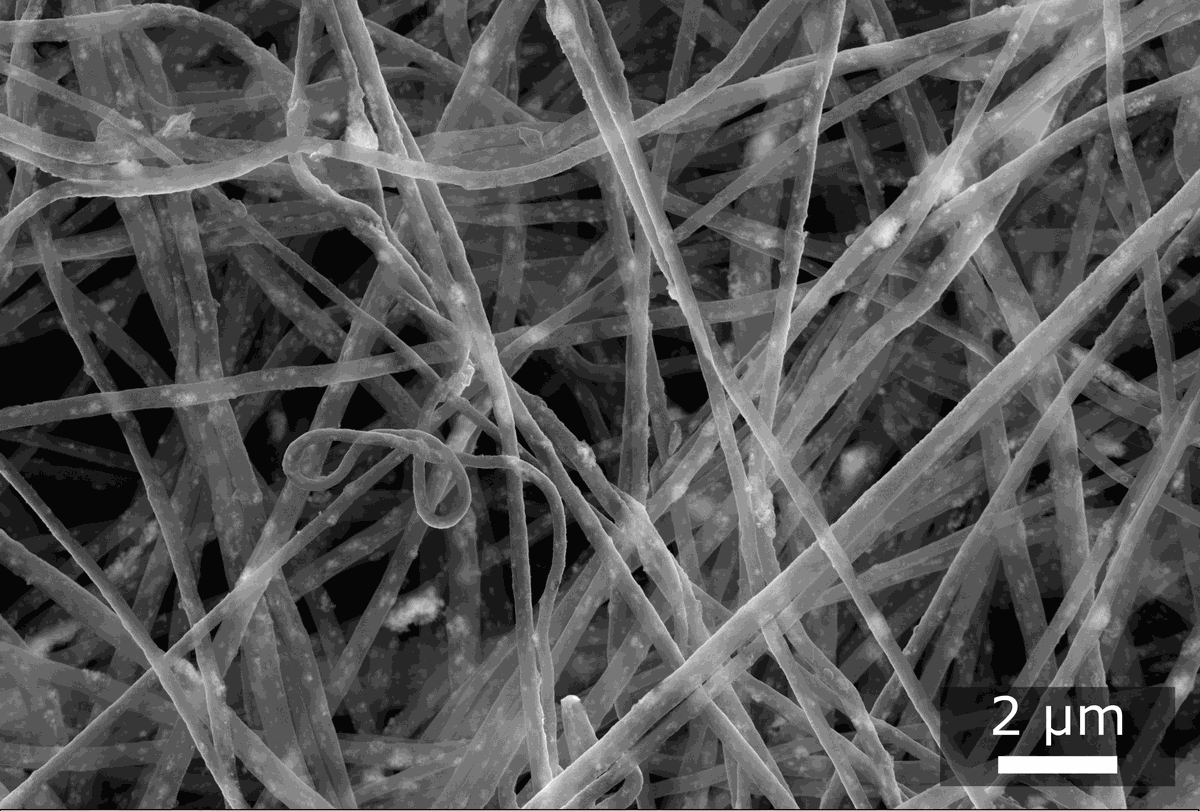
Electrospinning is used for the fabrication of nanofibers (polymer fibers with a diameter of less than 1 µm). In this technique, a polymer solution is dispensed via a needle onto a substrate where a fibrous mesh is formed. A strong electric field between the needle and the substrate (about 10 to 20 kV) accelerates the solution jet, which dries during the flight toward the substrate. We employ a needle-based solution electrospinning setup from KatoTech.
The nanofibrous meshes can be produced from single polymers, polymer blends, or polymer solutions with various additives. In the context of composite membranes, the resulting nanofiber meshes can be integrated into the membrane, for example, as a mechanical reinforcement or as chemically modified interlayers with specific functional groups.
Membrane electrode assemblies
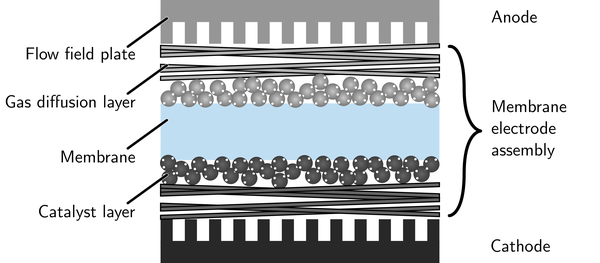
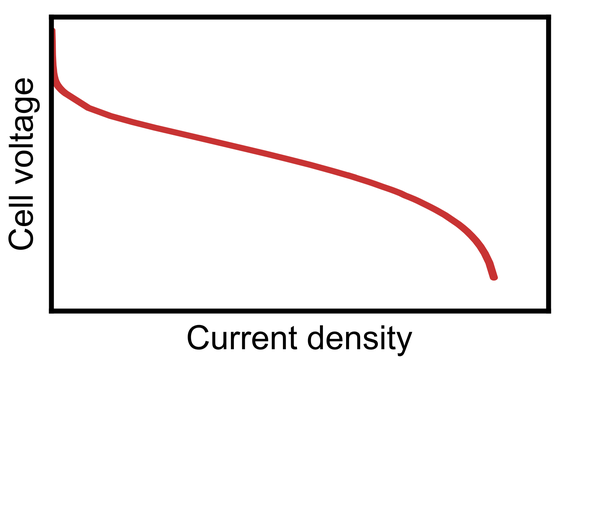
After the production of catalyst layers and membranes, the different layers need to be assembled to form a membrane electrode assembly. This step is typically performed by hot-pressing: The layers are pressed together while applying heat. As a result, close interfacial contact is formed between the layers, which now form a catalyst-coated membrane and are referred to as a membrane electrode assembly together with the gas diffusion layers. Good interfaces between the layers are the key to achieving high performance of the final electrochemical cell, like a fuel cell or water electrolyzer, while all layers need to maintain their integrity to avoid gas leaks and electrical short circuits.
Finally, the full cell is prepared by assembling the membrane electrode assembly in between two flow field plates. In water electrolyzers, the diffusion media are termed porous transport layers instead of gas diffusion layers, which is their designation in fuel cells. Additionally, a sealing element is required to ensure that the cell can be operated leak-free. This single cell can then be operated to investigate its performance, longevity, and durability versus certain stress factors.